History of atomic theory
Template:Short description Template:Redirect Template:About Template:Pp-vandalism Template:Good article

Atomic theory is the scientific theory that matter is composed of particles called atoms. The definition of the word "atom" has changed over the years in response to scientific discoveries. Initially, it referred to a hypothetical concept of there being some fundamental particle of matter, too small to be seen by the naked eye, that could not be divided. Then the definition was refined to being the basic particles of the chemical elements, when chemists observed that elements seemed to combine with each other in ratios of small whole numbers. Then physicists discovered that these particles had an internal structure of their own and therefore perhaps did not deserve to be called "atoms", but renaming atoms would have been impractical by that point.
Atomic theory is one of the most important scientific developments in history, crucial to all the physical sciences. At the start of The Feynman Lectures on Physics, physicist and Nobel laureate Richard Feynman offers the atomic hypothesis as the single most prolific scientific concept.<ref name="feynmanleightonsands1963-atomic">Template:Harvnb "If, in some cataclysm, all [] scientific knowledge were to be destroyed [save] one sentence [...] what statement would contain the most information in the fewest words? I believe it is [...] that all things are made up of atoms – little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another ..."</ref>
Philosophical atomism
Template:Main Template:See also The basic idea that matter is made up of tiny indivisible particles is an old idea that appeared in many ancient cultures. The word atom derived from the ancient Greek word atomos, a combination of the negative term "a-" and "τομή," the term for "cut", means "uncuttable".<ref name="Deutsch-1945">Template:Cite journal</ref> This ancient idea was based in philosophical reasoning rather than scientific reasoning. Modern atomic theory is not based on these old concepts.<ref name=Pullman-1998>Template:Cite book</ref><ref>Melsen (1952). From Atomos to Atom, pp. 18–19</ref> In the early 19th century, the scientist John Dalton noticed that chemical substances seemed to combine with each other by discrete and consistent units of weight, and he decided to use the word atom to refer to these units.<ref name="Pullman 1998 p. 201">Pullman (1998). The Atom in the History of Human Thought, p. 201</ref>
Groundwork
Working in the late 17th century, Robert Boyle developed the concept of a chemical element as substance different from a compound.<ref name="Whittaker"/>Template:Rp Near the end of the 18th century, a number of important developments in chemistry emerged without referring to the notion of an atomic theory. The first was Antoine Lavoisier who showed that compounds consist of elements in constant proportion, redefining an element as a substance which scientists could not decompose into simpler substances by experimentation. This brought an end to the ancient idea of the elements of matter being fire, earth, air, and water, which had no experimental support. Lavoisier showed that water can be decomposed into hydrogen and oxygen, which in turn he could not decompose into anything simpler, thereby proving these are elements.<ref>Pullman (1998). The Atom in the History of Human Thought. p. 197</ref> Lavoisier also defined the law of conservation of mass, which states that in a chemical reaction, matter does not appear nor disappear into thin air; the total mass remains the same even if the substances involved were transformed.<ref name="Whittaker"/>Template:Rp Finally, there was the law of definite proportions, established by the French chemist Joseph Proust in 1797, which states that if a compound is broken down into its constituent chemical elements, then the masses of those constituents will always have the same proportions by weight, regardless of the quantity or source of the original compound. This definition distinguished compounds from mixtures.<ref>Template:Cite web</ref>
Template:AnchorDalton's law of multiple proportions

John Dalton studied data gathered by himself and by other scientists. He noticed a pattern that later came to be known as the law of multiple proportions: in compounds which contain two particular elements, the amount of Element A per measure of Element B will differ across these compounds by ratios of small whole numbers. This suggested that each element combines with other elements in multiples of a basic quantity.<ref name="Pullman 1998 p. 199">Pullman (1998). The Atom in the History of Human Thought, p. 199: "The constant ratios, expressible in terms of integers, of the weights of the constituents in composite bodies could be construed as evidence on a macroscopic scale of interactions at the microscopic level between basic units with fixed weights. For Dalton, this agreement strongly suggested a corpuscular structure of matter, even though it did not constitute definite proof."</ref>
In 1804, Dalton explained his atomic theory to his friend and fellow chemist Thomas Thomson, who published the first full explanation chemical atomic theory in his book A System of Chemistry in 1807.<ref>Template:Cite book</ref>Template:Rp According to Thomson, Dalton's idea first occurred to him when experimenting with "olefiant gas" (ethylene) and "carburetted hydrogen gas" (methane). Dalton found that "carburetted hydrogen gas" contains twice as much hydrogen per measure of carbon as "olefiant gas", and concluded that a molecule of "olefiant gas" is one carbon atom and one hydrogen atom, and a molecule of "carburetted hydrogen gas" is one carbon atom and two hydrogen atoms.<ref>Thomas Thomson (1831). A History of Chemistry, Volume 2. p. 291</ref> In reality, an ethylene molecule has two carbon atoms and four hydrogen atoms (C2H4), and a methane molecule has one carbon atom and four hydrogen atoms (CH4).Template:Cn In this particular case, Dalton was mistaken about the formulas of these compounds, but he got the oxides of tin, iron, and nitrogen correct.<ref>Millington (1906). John Dalton, p. 113</ref>
Dalton defined an atom as being the "ultimate particle" of a chemical substance, and he used the term "compound atom" to refer to "ultimate particles" which contain two or more elements. This is inconsistent with the modern definition, wherein an atom is the basic particle of a chemical element and a molecule is an agglomeration of atoms. The term "compound atom" was confusing to some of Dalton's contemporaries as the word "atom" implies indivisibility, but he responded that if a carbon dioxide "atom" is divided, it ceases to be carbon dioxide. The carbon dioxide "atom" is indivisible in the sense that it cannot be divided into smaller carbon dioxide particles.<ref name="Pullman 1998 p. 201"/><ref>Dalton, quoted in Freund (1904). The Study of Chemical Composition. p. 288: "I have chosen the word atom to signify these ultimate particles in preference to particle, molecule, or any other diminiutive term, because I conceive it is much more expressive; it includes in itself the notion of indivisible, which the other terms do not. It may, perhaps, be said that I extend the application of it too far when I speak of compound atoms; for instance, I call an ultimate particle of carbonic acid a compound atom. Now, though this atom may be divided, yet it ceases to become carbonic acid, being resolved by such division into charcoal and oxygen. Hence I conceive there is no inconsistency in speaking of compound atoms and that my meaning cannot be misunderstood."</ref>
Dalton made the following assumptions on how "elementary atoms" combined to form "compound atoms" (what we today refer to as molecules). When two elements can only form one compound, he assumed it was one atom of each, which he called a "binary compound". If two elements can form two compounds, the first compound is a binary compound and the second is a "ternary compound" consisting of one atom of the first element and two of the second. If two elements can form three compounds between them, then the third compound is a "quaternary" compound containing one atom of the first element and three of the second.<ref>Dalton (1817). A New System of Chemical Philosophy vol. 1, pp. 213–214</ref> Dalton thought that water was a "binary compound", i.e. one hydrogen atom and one oxygen atom. Dalton did not know that in their natural gaseous state, the ultimate particles of oxygen, nitrogen, and hydrogen exist in pairs (O2, N2, and H2). Nor was he aware of valencies. These properties of atoms were discovered later in the 19th century.Template:Citation needed
Because atoms were too small to be directly weighed using the methods of the 19th century, Dalton instead expressed the weights of the myriad atoms as multiples of the hydrogen atom's weight, which Dalton knew was the lightest element. By his measurements, 7 grams of oxygen will combine with 1 gram of hydrogen to make 8 grams of water with nothing left over, and assuming a water molecule to be one oxygen atom and one hydrogen atom, he concluded that oxygen's atomic weight is 7. In reality it is 16. Aside from the crudity of early 19th century measurement tools, the main reason for this error was that Dalton didn't know that the water molecule in fact has two hydrogen atoms, not one. Had he known, he would have doubled his estimate to a more accurate 14. This error was corrected in 1811 by Amedeo Avogadro. Avogadro proposed that equal volumes of any two gases, at equal temperature and pressure, contain equal numbers of molecules (in other words, the mass of a gas's particles does not affect the volume that it occupies).<ref name="avogadro">Template:Cite journal</ref> Avogadro's hypothesis, now usually called Avogadro's law, provided a method for deducing the relative weights of the molecules of gaseous elements, for if the hypothesis is correct relative gas densities directly indicate the relative weights of the particles that compose the gases. This way of thinking led directly to a second hypothesis: the particles of certain elemental gases were pairs of atoms, and when reacting chemically these molecules often split in two. For instance, the fact that two liters of hydrogen will react with just one liter of oxygen to produce two liters of water vapor (at constant pressure and temperature) suggested that a single oxygen molecule splits in two in order to form two molecules of water. The formula of water is H2O, not HO. Avogadro measured oxygen's atomic weight to be 15.074.<ref>Template:Cite journal English translation</ref>
Opposition to atomic theory
Dalton's atomic theory attracted widespread interest but not everyone accepted it at first. The law of multiple proportions was shown not to be a universal law when it came to organic substances, whose molecules can be quite large. For instance, in oleic acid there is 34 g of hydrogen for every 216 g of carbon, and in methane there is 72 g of hydrogen for every 216 g of carbon. 34 and 72 form a ratio of 17:36, which is not a ratio of small whole numbers. We know now that carbon-based substances can have very large molecules, larger than any the other elements can form. Oleic acid's formula is C18H34O2 and methane's is CH4.<ref>Trusted (1999). The Mystery of Matter, p. 73</ref> The law of multiple proportions by itself was not complete proof, and atomic theory was not universally accepted until the end of the 19th century.<ref name="Pullman 1998 p. 199"/>
One problem was the lack of uniform nomenclature. The word "atom" implied indivisibility, but Dalton defined an atom as being the ultimate particle of any chemical substance, not just the elements or even matter per se. This meant that "compound atoms" such as carbon dioxide could be divided, as opposed to "elementary atoms". Dalton disliked the word "molecule", regarding it as "diminutive".<ref name="Pullman 1998 p. 201"/><ref>Freund (1904). The Study of Chemical Composition. p. 288</ref> Amedeo Avogadro did the opposite: he exclusively used the word "molecule" in his writings, eschewing the word "atom", instead using the term "elementary molecule".<ref>Pullman (1998). The Atom in the History of Human Thought, p. 202</ref> Jöns Jacob Berzelius used the term "organic atoms" to refer to particles containing three or more elements, because he thought this only existed in organic compounds. Jean-Baptiste Dumas used the terms "physical atoms" and "chemical atoms"; a "physical atom" was a particle that cannot be divided by physical means such as temperature and pressure, and a "chemical atom" was a particle that could not be divided by chemical reactions.<ref>Jean-Baptiste Dumas (1836). Leçons sur la philosophie chimique [Lessons on Chemical Philosophy]. 285–287</ref>
The modern definitions of atom and molecule—an atom being the basic particle of an element, and a molecule being an agglomeration of atoms—were established in the latter half of the 19th century. A key event was the Karlsruhe Congress in Germany in 1860. As the first international congress of chemists, its goal was to establish some standards in the community. A major proponent of the modern distinction between atoms and molecules was Stanislao Cannizzaro.
Cannizzaro criticized past chemists such as Berzelius for not accepting that the particles of certain gaseous elements are actually pairs of atoms, which led to mistakes in their formulation of certain compounds. Berzelius believed that hydrogen gas and chlorine gas particles are solitary atoms. But he observed that when one liter of hydrogen reacts with one liter of chlorine, they form two liters of hydrogen chloride instead of one. Berzelius decided that Avogadro's law does not apply to compounds. Cannizzaro preached that if scientists just accepted the existence of single-element molecules, such discrepancies in their findings would be easily resolved. But Berzelius did not even have a word for that. Berzelius used the term "elementary atom" for a gas particle which contained just one element and "compound atom" for particles which contained two or more elements, but there was nothing to distinguish H2 from H since Berzelius did not believe in H2. So Cannizzaro called for a redefinition so that scientists could understand that a hydrogen molecule can split into two hydrogen atoms in the course of a chemical reaction.<ref>Template:Cite book</ref>Template:Rp
A second objection to atomic theory was philosophical. Scientists in the 19th century had no way of directly observing atoms. They inferred the existence of atoms through indirect observations, such as Dalton's law of multiple proportions. Some scientists adopted positions aligned with the philosophy of positivism, arguing that scientists should not attempt to deduce the deeper reality of the universe, but only systemize what patterns they could directly observe.<ref name=Pullman-1998/>Template:Rp
This generation of anti-atomists can be grouped in two camps. The "equivalentists", like Marcellin Berthelot, believed the theory of equivalent weights was adequate for scientific purposes. This generalization of Proust's law of definite proportions summarized observations. For example, 1 gram of hydrogen will combine with 8 grams of oxygen to form 9 grams of water, therefore the "equivalent weight" of oxygen is 8 grams. The "energeticist", like Ernst Mach and Wilhelm Ostwald, were philosophically opposed to hypothesis about reality altogether. In their view, only energy as part of thermodynamics should be the basis of physical models.<ref name=Pullman-1998/>Template:Rp
These positions were eventually quashed by two important advancements that happened later in the 19th century: the development of the periodic table and the discovery that molecules have an internal architecture that determines their properties.<ref>Pullman (1998). The Atom in the History of Human Thought, p. 226: "The first development is the establishment of the periodic classification of the elements, marking the successful climax of concerted efforts to arrange the chemical properties of elements according to their atomic weight. The second is the emergence of structural chemistry, which ousted what was a simple and primitive verbal description of the elemental composition, be it atomic or equivalentist, of substances and replaced it with a systematic determination of their internal architecture."</ref>
Isomerism
Scientists discovered some substances have the exact same chemical content but different properties. For instance, in 1827, Friedrich Wöhler discovered that silver fulminate and silver cyanate are both 107 parts silver, 12 parts carbon, 14 parts nitrogen, and 16 parts oxygen (we now know their formulas as both AgCNO). In 1830 Jöns Jacob Berzelius introduced the term isomerism to describe the phenomenon. In 1860, Louis Pasteur hypothesized that the molecules of isomers might have the same set of atoms but in different arrangements.<ref>Pullman (1998). The Atom in the History of Human Thought, p. 230</ref>
In 1874, Jacobus Henricus van 't Hoff proposed that the carbon atom bonds to other atoms in a tetrahedral arrangement. Working from this, he explained the structures of organic molecules in such a way that he could predict how many isomers a compound could have. Consider, for example, pentane (C5H12). In van 't Hoff's way of modelling molecules, there are three possible configurations for pentane, and scientists did go on to discover three and only three isomers of pentane.<ref>Melsen (1952). From Atomos to Atom, pp. 147–148</ref><ref>Henry Enfield Roscoe, Carl Schorlemmer (1895). A Treatise on Chemistry, Volume 3, Part 1, pp. 121–122</ref>
Isomerism was not something that could be fully explained by alternative theories to atomic theory, such as radical theory and the theory of types.<ref>Henry Enfield Roscoe, Carl Schorlemmer (1895). A Treatise on Chemistry, Volume 3, Part 1, pp. 121: "The radical theory and the theory of types are capable of explaining many cases of isomerism, but it was not until the doctrine of the linking of atoms was established that a clear light was thrown on this subject."</ref><ref>Adolphe Wurtz (1880). The Atomic Theory, p. 291: "It is in this manner that the theory of atomicity predicts, interprets, and limits the number of isomers; it has furnished the elements of one of the greatest advances which science has accomplished in the last twenty years. [...] The theory of atomicity has successfully attacked the problem by introducing into the discussion exact data, which have been in a great number of cases confirmed by experiment."</ref>
Mendeleev's periodic table
Template:Main Dmitrii Mendeleev noticed that when he arranged the elements in a row according to their atomic weights, there was a certain periodicity to them.<ref name="Scerri">Template:Cite book</ref>Template:Rp For instance, the second element, lithium, had similar properties to the ninth element, sodium, and the sixteenth element, potassium — a period of seven. Likewise, beryllium, magnesium, and calcium were similar and all were seven places apart from each other on Mendeleev's table. Using these patterns, Mendeleev predicted the existence and properties of new elements, which were later discovered in nature: scandium, gallium, and germanium.<ref name="Scerri"/>Template:Rp Moreover, the periodic table could predict how many atoms of other elements that an atom could bond with — e.g., germanium and carbon are in the same group on the table and their atoms both combine with two oxygen atoms each (GeO2 and CO2). Mendeleev found these patterns validated atomic theory because it showed that the elements could be categorized by their atomic weight. Inserting a new element into the middle of a period would break the parallel between that period and the next, and would also violate Dalton's law of multiple proportions.<ref>Template:Cite journal</ref>

The elements on the periodic table were originally arranged in order of increasing atomic weight. However, in a number of places chemists chose to swap the positions of certain adjacent elements so that they appeared in a group with other elements with similar properties. For instance, tellurium is placed before iodine even though tellurium is heavier (127.6 vs 126.9) so that iodine can be in the same column as the other halogens. The modern periodic table is based on atomic number, which is equivalent to the nuclear charge, a change that had to wait for the discovery of the nucleus.<ref name="PaisInwardBound"/>Template:Rp In addition, an entire row of the table was not shown because the noble gases had not been discovered when Mendeleev devised his table.<ref name="PaisInwardBound"/>Template:Rp
Kinetic theory of gases
In 1738, Swiss physicist and mathematician Daniel Bernoulli postulated that the pressure of gases and heat were both caused by the underlying motion of molecules. Using his model he could predict the ideal gas law at constant temperature and suggested that the temperature was proportional to the velocity of the particles. This success was not followed up, in part because the then new tools of calculus allowed more progress using continuous models for gases.<ref name="Uffink-2006">Template:Cite book</ref>Template:Rp
James Clerk Maxwell, a vocal proponent of atomism, revived the kinetic theory in 1860 and 1867. His key insight was that the velocity of particles in a gas would vary around an average value, introducing the concept of a distribution function.<ref name="Uffink-2006"/>Template:Rp<ref>See:
- Maxwell, J.C. (1860) "Illustrations of the dynamical theory of gases. Part I. On the motions and collisions of perfectly elastic spheres," Philosophical Magazine, 4th series, 19 : 19–32.
- Maxwell, J.C. (1860) "Illustrations of the dynamical theory of gases. Part II. On the process of diffusion of two or more kinds of moving particles among one another," Philosophical Magazine, 4th series, 20 : 21–37.</ref> In the late 1800s, Ludwig Boltzmann used atomic models to apply kinetic theory to thermodynamics especially the second law relating to entropy. Boltzmann defended the atomistic hypothesis against major detractors from the time like Ernst Mach or energeticists like Wilhelm Ostwald, who considered that energy was the elementary quantity of reality.<ref>Template:Cite journal</ref> However an atomic model was not essential for the development of theory of thermodynamics. This became clear when Josiah Willard Gibbs introduced statistical mechanics in his 1902 book Elementary Principles in Statistical Mechanics. His logical and formal development of a new approach specifically avoided requiring an atomic hypothesis.<ref name="Uffink-2006"/>Template:Rp Albert Einstein independently developed an approach similar to Gibbs, but with a completely different aim: Einstein set out to find a way to verify the atomic hypothesis through the kinetic theory. He would eventually succeed with a paper on Brownian motion.<ref name="Bernstein-2006">Template:Cite journal</ref>
Brownian motion
In 1827, the British botanist Robert Brown observed that dust particles inside pollen grains floating in water constantly jiggled about for no apparent reason. In 1905, Einstein theorized that this Brownian motion was caused by the water molecules continuously knocking the grains about, and developed a mathematical model to describe it.<ref name="Bernstein-2006"/> This model was validated experimentally in 1908 by French physicist Jean Perrin, who used Einstein's equations to measure the size of atoms.<ref>Template:Cite web</ref>
| Molecule | Perrin's measurements<ref name="Perrin 1909 p. 50">Perrin (1909). Brownian Movement and Molecular Reality, p. 50</ref> | Modern measurements |
|---|---|---|
| Helium | 1.7 × 10−10 m | 2.6 × 10−10 m |
| Argon | 2.7 × 10−10 m | 3.4 × 10−10 m |
| Mercury | 2.8 × 10−10 m | 3 × 10−10 m |
| Hydrogen | 2 × 10−10 m | 2.89 × 10−10 m |
| Oxygen | 2.6 × 10−10 m | 3.46 × 10−10 m |
| Nitrogen | 2.7 × 10−10 m | 3.64 × 10−10 m |
| Chlorine | 4 × 10−10 m | 3.20 × 10−10 m |
| Molecule | Perrin's measurements<ref name="Perrin 1909 p. 50"/> | Modern measurements |
|---|---|---|
| Hydrogen | 1.43 × 10−27 kg | 1.66 × 10−27 kg |
| Oxygen | 22.7 × 10−27 kg | 22.8 × 10−27 kg |
Plum pudding model
Template:Main Template:Multiple image
Atoms were thought to be the smallest possible division of matter until 1899 when J. J. Thomson discovered the electron through his work on cathode rays.<ref name="PaisInwardBound"/>Template:Rp<ref name="Whittaker">Template:Cite book</ref>Template:Rp
A Crookes tube is a sealed glass container in which two electrodes are separated by a vacuum. When a voltage is applied across the electrodes, cathode rays are generated, creating a glowing patch where they strike the glass at the opposite end of the tube. Through experimentation, Thomson discovered that the rays could be deflected by electric fields and magnetic fields, which meant that these rays were not a form of light but were composed of very light charged particles, and their charge was negative. Thomson called these particles "corpuscles". He measured their mass-to-charge ratio to be several orders of magnitude smaller than that of the hydrogen atom, the smallest atom. This ratio was the same regardless of what the electrodes were made of and what the trace gas in the tube was.<ref name="thomson">Template:Cite journal
"From these determinations we see that the value of m/e is independent of the nature of the gas, and that its value 10−7 is very small compared with the value 10−4, which is the smallest value of this quantity previously known, and which is the value for the hydrogen ion in electrolysis."</ref>
In contrast to those corpuscles, positive ions created by electrolysis or X-ray radiation had mass-to-charge ratios that varied depending on the material of the electrodes and the type of gas in the reaction chamber, indicating they were different kinds of particles.<ref name="Whittaker"/>Template:Rp
In 1898, Thomson measured the charge on ions to be roughly 6 × 10−10 electrostatic units (2 × 10−19 Coulombs).<ref name="PaisInwardBound">Template:Cite book</ref>Template:Rp<ref>Template:Cite journal</ref> In 1899, he showed that negative electricity created by ultraviolet light landing on a metal (known now as the photoelectric effect) has the same mass-to-charge ratio as cathode rays; then he applied his previous method for determining the charge on ions to the negative electric particles created by ultraviolet light.<ref name="PaisInwardBound"/>Template:Rp By this combination he showed that electron's mass was 0.0014 times that of hydrogen ions.<ref>Template:Cite journal
"...the magnitude of this negative charge is about 6 × 10−10 electrostatic units, and is equal to the positive charge carried by the hydrogen atom in the electrolysis of solutions. [...] In gases at low pressures these units of negative electric charge are always associated with carriers of a definite mass. This mass is exceedingly small, being only about 1.4 × 10−3 of that of the hydrogen ion, the smallest mass hitherto recognized as capable of a separate existence. The production of negative electrification thus involves the splitting up of an atom, as from a collection of atoms something is detached whose mass is less than that of a single atom."</ref> These "corpuscles" were so light yet carried so much charge that Thomson concluded they must be the basic particles of electricity, and for that reason other scientists decided that these "corpuscles" should instead be called electrons following an 1894 suggestion by George Johnstone Stoney for naming the basic unit of electrical charge.<ref>Template:Cite book</ref>
In 1904, Thomson published a paper describing a new model of the atom.<ref>Template:Cite journal</ref> Electrons reside within atoms, and they transplant themselves from one atom to the next in a chain in the action of an electrical current. When electrons do not flow, their negative charge logically must be balanced out by some source of positive charge within the atom so as to render the atom electrically neutral. Having no clue as to the source of this positive charge, Thomson tentatively proposed that the positive charge was everywhere in the atom, the atom being shaped like a sphere—this was the mathematically simplest model to fit the available evidence (or lack of it).<ref>J. J. Thomson (1907). The Corpuscular Theory of Matter, p. 103: "In default of exact knowledge of the nature of the way in which positive electricity occurs in the atom, we shall consider a case in which the positive electricity is distributed in the way most amenable to mathematical calculation, i.e., when it occurs as a sphere of uniform density, throughout which the corpuscles are distributed."</ref> The balance of electrostatic forces would distribute the electrons throughout this sphere in a more or less even manner. Thomson further explained that ions are atoms that have a surplus or shortage of electrons.<ref>J. J. Thomson (1907). On the Corpuscular Theory of Matter, p. 26: "The simplest interpretation of these results is that the positive ions are the atoms or groups of atoms of various elements from which one or more corpuscles have been removed. That, in fact, the corpuscles are the vehicles by which electricity is carried from one body to another, a positively electrified body different from the same body when unelectrified in having lost some of its corpuscles while the negative electrified body is one with more corpuscles than the unelectrified one."</ref>
Thomson's model is popularly known as the plum pudding model, based on the idea that the electrons are distributed throughout the sphere of positive charge with the same density as raisins in a plum pudding. Neither Thomson nor his colleagues ever used this analogy. It seems to have been a conceit of popular science writers.<ref>Template:Cite journal</ref> The analogy suggests that the positive sphere is like a solid, but Thomson likened it to a jelly, as he proposed that the electrons moved around in it in patterns governed by the electrostatic forces.<ref>J. J. Thomson, in a letter to Oliver Lodge dated 11 April 1904, quoted in Davis & Falconer (1997):
"With regard to positive electrification I have been in the habit of using the crude analogy of a liquid with a certain amount of cohesion, enough to keep it from flying to bits under its own repulsion. I have however always tried to keep the physical conception of the positive electricity in the background because I have always had hopes (not yet realised) of being able to do without positive electrification as a separate entity and to replace it by some property of the corpuscles."
</ref><ref name=Heilbron1968/>Template:Rp The positive electrification in Thomson's model was a temporary concept, which he hoped would ultimately be explained by some phenomena of the electrons. Like all atomic models of that time, Thomson's model was incomplete, it could not predict any of the known properties of the atom such as emission spectra.<ref name="Kragh-2010"/>
In 1910, Robert A. Millikan and Harvey Fletcher reported the results of their oil drop experiment in which they isolated and measured the charge of an electron.<ref>Template:Cite journal</ref> Careful measurements over several years gave the charge -4.774 × 10−10esu.<ref>Template:Cite journal</ref> Template:Clear
Planetary models
In the late 1800s speculations on the possible structure of the atom included planetary models with orbiting charged electrons.<ref name=Kragh2010>Helge Kragh (Oct. 2010). Before Bohr: Theories of atomic structure 1850-1913. RePoSS: Research Publications on Science Studies 10. Aarhus: Centre for Science Studies, University of Aarhus.</ref>Template:Rp These models faced a significant constraint. In 1897, Joseph Larmor showed that an accelerating charge would radiate power according to classical electrodynamics, a result known as the Larmor formula. Since electrons forced to remain in orbit are continuously accelerating, they would be mechanically unstable. Larmor noted that electromagnetic effect of multiple electrons, suitably arranged, would cancel each other. Thus subsequent atomic models based on classical electrodynamics needed to adopt such special multi-electron arrangements.<ref>Template:Cite book</ref>Template:Rp
Haas atomic model
In 1910, Arthur Erich Haas proposed a model of the hydrogen atom with an electron circulating on the surface of a sphere of positive charge. The model resembled Thomson's plum pudding model, but Haas added a radical new twist: he constrained the electron's potential energy, <math>E_\text{pot}</math>, on a sphere of radius Template:Mvar to equal the frequency, Template:Mvar, of the electron's orbit on the sphere times the Planck constant:<ref name=PaisInwardBound/>Template:Rp <math display="block">E_\text{pot}= \frac{- e^2}{a} = hf </math> where Template:Mvar represents the charge on the electron and the sphere. Haas combined this constraint with the balance-of-forces equation. The attractive force between the electron and the sphere balances the centrifugal force: <math display="block">\frac{e^2}{a^2} = ma(2\pi f)^2</math> where Template:Mvar is the mass of the electron. This combination relates the radius of the sphere to the Planck constant: <math display="block">a = \frac{h^2}{4\pi^2e^2m}</math> Haas solved for the Planck constant using the then-current value for the radius of the hydrogen atom. Three years later, Bohr would use similar equations with different interpretation. Bohr took the Planck constant as given value and used the equations to predict, Template:Mvar, the radius of the electron orbiting in the ground state of the hydrogen atom. This value is now called the Bohr radius.<ref name=PaisInwardBound/>Template:Rp
Nicholson atom theory
In 1911 John William Nicholson published a model of the atom similar to JJ Thomson's but with a positive charge radius shrunk from atomic to below the radius of his electrons. Nicholson developed his model based on the analysis of astrophysical spectroscopy. He connected the observed spectral line frequencies with the orbits of electrons in his atoms. The connection he adopted associated the atomic electron orbital angular momentum with the Planck constant. Whereas Planck focused on a quantum of energy, Nicholson's angular momentum quantum relates to orbital frequency. This new concept gave Planck constant an atomic meaning for the first time.<ref name="McCormmach1966">Template:Cite journal</ref>Template:Rp In his 1913 paper Bohr cites Nicholson as finding quantized angular momentum important for the atom.<ref name="bohr1">Template:Cite journal</ref>
Nicholson's model was based on classical electrodynamics along the lines of J.J. Thomson's plum pudding model but his negative electrons orbiting a positive nucleus rather than circulating in a sphere. To avoid immediate collapse of this system he required that electrons come in pairs so the rotational acceleration of each electron was matched across the orbit.<ref name="McCormmach1966"/>Template:Rp By 1913 Bohr had already shown, from the analysis of alpha particle energy loss, that hydrogen had only a single electron not a matched pair.<ref name=PaisInwardBound/>Template:Rp Bohr's atomic model would abandon classical electrodynamics.
Nicholson's model of radiation was quantum but was attached to the orbits of the electrons.<ref name="Nicholson1912">Template:Cite journal</ref><ref name="Heilbron2013">Template:Cite journal</ref> Template:Rp Bohr quantization would associate it with differences in energy levels of his model of hydrogen rather than the orbital frequency.
Discovery of the nucleus

Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
Thomson's plum pudding model was challenged in 1911 by one of his former students, Ernest Rutherford, who presented a new model to explain new experimental data. The new model proposed a concentrated center of charge and mass that was later dubbed the atomic nucleus.<ref name=Heilbron1968>Template:Cite journal</ref>Template:Rp
Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to have doubts about the Thomson model after they encountered difficulties when they tried to build an instrument to measure the charge-to-mass ratio of alpha particles (these are positively-charged particles emitted by certain radioactive substances such as radium). The alpha particles were being scattered by the air in the detection chamber, which made the measurements unreliable. Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments. Rutherford didn't think he'd run into this same problem because alpha particles usually have much more momentum than electrons. According to Thomson's model of the atom, the positive charge in the atom is not concentrated enough to produce an electric field strong enough to deflect an alpha particle. Yet there was scattering, so Rutherford and his colleagues decided to investigate this scattering carefully.<ref name=Heilbron2003p64-68>Heilbron (2003). Ernest Rutherford and the Explosion of Atoms, pp. 64–68Template:Broken anchor</ref>
Between 1908 and 1913, Rutherford and his colleagues performed a series of experiments in which they bombarded thin foils of metal with a beam of alpha particles. They spotted alpha particles being deflected by angles greater than 90°. According to Thomson's model, all of the alpha particles should have passed through with negligible deflection. Rutherford deduced that the positive charge of the atom is not distributed throughout the atom's volume as Thomson believed, but is concentrated in a tiny nucleus at the center. This nucleus also carries most of the atom's mass. Only such an intense concentration of charge, anchored by its high mass, could produce an electric field strong enough to deflect the alpha particles as observed.<ref name=Heilbron2003p64-68/> Rutherford's model, being supported primarily by scattering data unfamiliar to many scientists, did not catch on until Niels Bohr joined Rutherford's lab and developed a new model for the electrons.<ref name=Heilbron1968/>Template:Rp
Rutherford model predicted that the scattering of alpha particles would be proportional to the square of the atomic charge. Geiger and Marsden's based their analysis on setting the charge to half of the atomic weight of the foil's material (gold, aluminium, etc.). Amateur physicist Antonius van den Broek noted that there was a more precise relation between the charge and the element's numeric sequence in the order of atomic weights. The sequence number came be called the atomic number and it replaced atomic weight in organizing the periodic table.<ref>Template:Cite journal</ref><ref>Template:Cite journal</ref> Template:Clear
Discovery of isotopes
Template:Main Concurrent with the work of Rutherford, Geiger, and Marsden, the radiochemist Frederick Soddy at the University of Glasgow was studying chemistry-related problems on radioactive materials. Soddy had worked with Rutherford on radioactivity at McGill University.<ref name="Nobel">Template:Cite web</ref> By 1910, about 40 different radioactive elements, referred to as radioelements, had been identified between uranium and lead, although the periodic table only allowed for 11 elements. Every attempt to chemically isolate the radioelements mesothorium or thorium X from radium failed. Soddy concluded that these element were chemically the same element. At the suggestion of Margaret Todd, Soddy called these chemically identical elements isotopes.<ref>Template:Cite journal</ref><ref> Template:Citation</ref>Template:Rp In 1913, Soddy and theorist Kazimierz Fajans independently found the displacement law, that an element undergoing alpha decay will produce an element two places to the left in the periodic system and an element undergoing beta decay will produce an element one place to the right in the periodic system. For his study of radioactivity and the discovery of isotopes, Soddy was awarded the 1921 Nobel Prize in Chemistry.<ref>Template:Cite web</ref>

Prior to 1919 only atomic weights averaged over a very large number of atoms was available. In that year, Francis Aston built the first mass spectrograph, an improved form of a device built by J. J. Thomson to measure the deflection of positively charged atoms by electric and magnetic fields. Aston was then able to separate the isotopes of many light elements including neon, Template:SimpleNuclide and Template:SimpleNuclide. Aston discovered the isotopes matched William Prout's whole number rule: the mass of every isotope is a whole number multiple of hydrogen.<ref>Aston, Francis William. Mass spectra and isotopes. London: Edward Arnold, 1942.</ref><ref name="Squires-1998">Template:Cite journal</ref>
Significantly, the one exception to this whole number rule was hydrogen itself, which had a mass value of 1.008. The excess mass was small, but well outside the limits of experimental uncertainty. Aston and others realized this difference was due to the binding energy of atoms. When a number of hydrogen atoms are bound into a atom, that atom's energy must be less than the sum of the energies of the separate hydrogen atoms. That lost energy, according to the mass-energy equivalence principle, means the atomic mass will be slightly less than the sum of the masses of its components.<ref name='Squires-1998'/> Aston's work on isotopes won him the 1922 Nobel Prize in Chemistry for the discovery of isotopes in a large number of non-radioactive elements, and for his enunciation of the whole number rule.<ref>Template:Cite web</ref>
Atomic number
Before 1913, chemists adhered to Mendeleev's principle that chemical properties derived from atomic weight. However, several places in the periodic table were inconsistent with this concept. For example cobalt and nickel seemed reversed.<ref name="Heilbron"> Template:Cite book</ref>Template:Rp There were also attempts to understand the relationship between the atomic mass and nuclear charge. Rutherford knew from experiments in his lab that helium must have a nuclear charge of 2 and a mass of 4; this 1:2 ratio was expected to hold for all elements. In 1913 Antonius van den Broek hypothesized that the periodic table should be organized by charge, denoted by Z, not atomic mass and that Z was not exactly half of the atomic weight for elements.<ref name="PaisInwardBound"/>Template:Rp This solved the cobalt-nickel issue. Placing cobalt (Z=27, mass of 58.97), before the heavier nickel (Z=28, mass of 58.68) gave the ordering expected by chemical behavior.<ref name="Pais1993">Template:Cite book</ref>Template:Rp
In 1913–1914 Moseley tested Broek's hypothesis experimentally by using X-ray spectroscopy. He found that the most intense short-wavelength line in the X-ray spectrum of a particular element, known as the K-alpha line, was related to the element's charge its atomic number, Z.<ref>Template:Cite journal</ref> Moseley found that the frequencies of the radiation were related in a simple way to the atomic number of the elements for a large number of elements.<ref>Template:Cite journal</ref><ref name=Pais1993/>Template:Rp
Bohr model
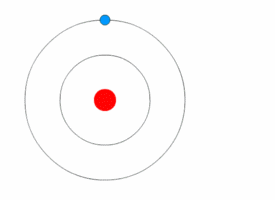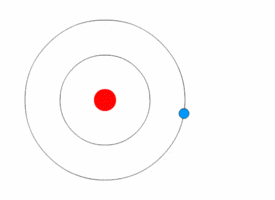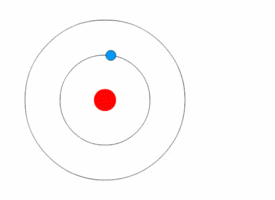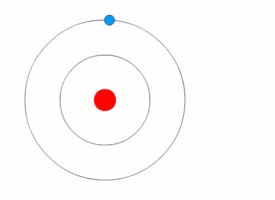
Rutherford deduced the existence of the atomic nucleus through his experiments but he had nothing to say about how the electrons were arranged around it. In 1912, Niels Bohr joined Rutherford's lab and began his work on a quantum model of the atom.<ref name=PaisInwardBound/>Template:Rp
Max Planck in 1900 and Albert Einstein in 1905 had postulated that light energy is emitted or absorbed in discrete amounts known as quanta (singular, quantum). This led to a series of atomic models with some quantum aspects, such as that of Arthur Erich Haas in 1910<ref name=PaisInwardBound/>Template:Rp and the 1912 John William Nicholson atomic model with quantized angular momentum as h/2Template:Pi.<ref>J. W. Nicholson, Month. Not. Roy. Astr. Soc. lxxii. pp. 49,130, 677, 693, 729 (1912).</ref><ref>The Atomic Theory of John William Nicholson, Russell McCormmach, Archive for History of Exact Sciences, Vol. 3, No. 2 (25.8.1966), pp. 160–184 (25 pages), Springer.</ref> The dynamical structure of these models was still classical.
In 1913, Bohr abandon the classical approach. He started his Bohr model of the atom with a quantum hypothesis: an electron could only orbit the nucleus in particular circular orbits with fixed angular momentum and energy, its distance from the nucleus (i.e., their radii) being proportional to its energy.<ref name=PaisInwardBound/>Template:Rp<ref name="NBohr">Template:Cite journal</ref> Under this model an electron could not lose energy in a continuous manner; instead, it could only make instantaneous "quantum leaps" between the fixed energy levels.<ref name="NBohr"/> When this occurred, light was emitted or absorbed at a frequency proportional to the change in energy (hence the absorption and emission of light in discrete spectra).<ref name="NBohr"/> In comparing his work to Nicholson's, Bohr came to understand the spectral data and their value. When he then learned from a friend about Balmer's compact formula for the spectral line data, Bohr quickly realized his model would match it in detail.<ref name="McCormmach1966"/>Template:Rp
In a trilogy of papers Bohr described and applied his model to derive the Balmer series of lines in the atomic spectrum of hydrogen and the related spectrum of He+.<ref name=PaisInwardBound/>Template:Rp He also used he model to describe the structure of the periodic table and aspects of chemical bonding. Together these results lead to Bohr's model being widely accepted by the end of 1915.<ref name=Kragh-2012>Template:Cite book</ref>Template:Rp
Bohr's model was not perfect. It could only predict the spectral lines of hydrogen, not those of multielectron atoms.<ref>Template:Cite journal</ref> Worse still, it could not even account for all features of the hydrogen spectrum: as spectrographic technology improved, it was discovered that applying a magnetic field caused spectral lines to multiply in a way that Bohr's model couldn't explain. In 1916, Arnold Sommerfeld added elliptical orbits to the Bohr model to explain the extra emission lines, but this made the model very difficult to use, and it still couldn't explain more complex atoms.<ref>Template:Cite book</ref><ref>Template:Cite journal</ref>
Discovery of the proton
Template:Prose Back in 1815, William Prout observed that the atomic weights of the known elements were multiples of hydrogen's atomic weight, so he hypothesized that all atoms are agglomerations of hydrogen, a particle which he dubbed "the protyle". Prout's hypothesis was put into doubt when some elements were found to deviate from this pattern—e.g. chlorine atoms on average weigh 35.45 daltons—but when isotopes were discovered in 1913, Prout's observation gained renewed attention.<ref name="Kragh-2010">Template:Cite web</ref>
In 1898, J. J. Thomson found that the positive charge of a hydrogen ion was equal to the negative charge of a single electron.<ref>Template:Cite journal</ref>
In an April 1911 paper concerning his studies on alpha particle scattering, Ernest Rutherford estimated that the charge of an atomic nucleus, expressed as a multiplier of hydrogen's nuclear charge (qe), is roughly half the atom's atomic weight.<ref>Template:Cite journal</ref>
In June 1911, Van den Broek noted that on the periodic table, each successive chemical element increased in atomic weight on average by 2, which in turn suggested that each successive element's nuclear charge increased by 1 qe.<ref>Template:Cite journal
"Hence, if this cubic periodic system should prove to be correct, then the number of possible elements is equal to the number of possible permanent charges of each sign per atom, or to each possible permanent charge (of both signs) per atom belongs a possible element."</ref> In 1913, van den Broek further proposed that the electric charge of an atom's nucleus, expressed as a multiplier of the elementary charge, is equal to the element's sequential position on the periodic table. Rutherford defined this position as being the element's atomic number.<ref>Eric Scerri (2020). The Periodic Table: Its Story and Its Significance, p. 185</ref><ref>Template:Cite journal</ref><ref>Template:Cite journal</ref>
In 1913, Henry Moseley measured the X-ray emissions of all the elements on the periodic table and found that the frequency of the X-ray emissions was a mathematical function of the element's atomic number and the charge of a hydrogen nucleus Template:Crossreference.Template:Citation needed
In 1917 Rutherford bombarded nitrogen gas with alpha particles and observed hydrogen ions being emitted from the gas. Rutherford concluded that the alpha particles struck the nuclei of the nitrogen atoms, causing hydrogen ions to split off.<ref>Template:Cite journal</ref><ref>The Development of the Theory of Atomic Structure (Rutherford 1936). Reprinted in Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936:
"In 1919 I showed that when light atoms were bombarded by α-particles they could be broken up with the emission of a proton, or hydrogen nucleus. We therefore presumed that a proton must be one of the units of which the nuclei of other atoms were composed..."</ref>
These observations led Rutherford to conclude that the hydrogen nucleus was a singular particle with a positive charge equal to that of the electron's negative charge. The name "proton" was suggested by Rutherford at an informal meeting of fellow physicists in Cardiff in 1920.<ref>Template:Cite journal
Footnote by Ernest Rutherford: 'At the time of writing this paper in Australia, Professor Orme Masson was not aware that the name "proton" had already been suggested as a suitable name for the unit of mass nearly 1, in terms of oxygen 16, that appears to enter into the nuclear structure of atoms. The question of a suitable name for this unit was discussed at an informal meeting of a number of members of Section A of the British Association [for the Advancement of Science] at Cardiff this year. The name "baron" suggested by Professor Masson was mentioned, but was considered unsuitable on account of the existing variety of meanings. Finally the name "proton" met with general approval, particularly as it suggests the original term "protyle" given by Prout in his well-known hypothesis that all atoms are built up of hydrogen. The need of a special name for the nuclear unit of mass 1 was drawn attention to by Sir Oliver Lodge at the Sectional meeting, and the writer then suggested the name "proton."'</ref>
The charge number of an atomic nucleus was found to be equal to the element's ordinal position on the periodic table. The nuclear charge number thus provided a simple and clear-cut way of distinguishing the chemical elements from each other, as opposed to Lavoisier's classic definition of a chemical element being a substance that cannot be broken down into simpler substances by chemical reactions. The charge number or proton number was thereafter referred to as the atomic number of the element. In 1923, the International Committee on Chemical Elements officially declared the atomic number to be the distinguishing quality of a chemical element.<ref>Template:Cite journal</ref>
During the 1920s, some writers defined the atomic number as being the number of "excess protons" in a nucleus. Before the discovery of the neutron, scientists believed that the atomic nucleus contained a number of "nuclear electrons" which cancelled out the positive charge of some of its protons. This explained why the atomic weights of most atoms were higher than their atomic numbers. Helium, for instance, was thought to have four protons and two nuclear electrons in the nucleus, leaving two excess protons and a net nuclear charge of 2+. After the neutron was discovered, scientists realized the helium nucleus in fact contained two protons and two neutrons.Template:Cn
Quantum mechanical models

In 1924, Louis de Broglie proposed that all particles—particularly subatomic particles such as electrons—have an associated wave. Erwin Schrödinger, fascinated by this idea, developed an equation<ref name="schrodinger">Template:Cite journal</ref> that describes an electron as a wave function instead of a point. This approach predicted many of the spectral phenomena that Bohr's model failed to explain, but it was difficult to visualize, and faced opposition.<ref name="Mahanti">Template:Cite news</ref> One of its critics, Max Born, proposed instead that Schrödinger's wave function did not describe the physical extent of an electron (like a charge distribution in classical electromagnetism), but rather gave the probability that an electron would, when measured, be found at a particular point.<ref>Template:Cite news</ref> This reconciled the ideas of wave-like and particle-like electrons: the behavior of an electron, or of any other subatomic entity, has both wave-like and particle-like aspects, and whether one aspect or the other is observed depend upon the experiment.<ref>Template:Cite news</ref>
Schrödinger's wave model for hydrogen replaced Bohr's model, with its neat, clearly defined circular orbits. The modern model of the atom describes the positions of electrons in an atom in terms of probabilities. An electron can potentially be found at any distance from the nucleus, but, depending on its energy level and angular momentum, exists more frequently in certain regions around the nucleus than others; this pattern is referred to as its atomic orbital. The orbitals come in a variety of shapes—sphere, dumbbell, torus, etc.—with the nucleus in the middle.<ref>Template:Cite news</ref> The shapes of atomic orbitals are found by solving the Schrödinger equation.<ref>Template:Cite book</ref> Analytic solutions of the Schrödinger equation are known for very few relatively simple model Hamiltonians including the hydrogen atom and the hydrogen molecular ion.<ref>Template:Cite journal</ref> Beginning with the helium atom—which contains just two electrons—numerical methods are used to solve the Schrödinger equation.<ref>Template:Cite journal</ref>
Qualitatively the shape of the atomic orbitals of multi-electron atoms resemble the states of the hydrogen atom. The Pauli principle requires the distribution of these electrons within the atomic orbitals such that no more than two electrons are assigned to any one orbital; this requirement profoundly affects the atomic properties and ultimately the bonding of atoms into molecules.<ref>Karplus, Martin, and Richard Needham Porter. "Atoms and molecules; an introduction for students of physical chemistry." Atoms and molecules; an introduction for students of physical chemistry (1970).</ref>Template:Rp
Discovery of the neutron
Template:Main Physicists in the 1920s believed that the atomic nucleus contained protons plus a number of "nuclear electrons" that reduced the overall charge. These "nuclear electrons" were distinct from the electrons that orbited the nucleus. This incorrect hypothesis would have explained why the atomic numbers of the elements were less than their atomic weights, and why radioactive elements emit electrons (beta radiation) in the process of nuclear decay. Rutherford even hypothesized that a proton and an electron could bind tightly together into a "neutral doublet". Rutherford wrote that the existence of such "neutral doublets" moving freely through space would provide a more plausible explanation for how the heavier elements could have formed in the genesis of the Universe, given that it is hard for a lone proton to fuse with a large atomic nucleus because of the repulsive electric field.<ref>Template:Cite journal: "Under some conditions, however, it may be possible for an electron to combine much more closely with the H nucleus, forming a kind of neutral doublet. [...] The existence of such atoms seems almost necessary to explain the building up of the nuclei of heavy elements; for unless we suppose the production of charged particles of very high velocities it is difficult to see how any positively charged particle can reach the nucleus of a heavy atom against its intense repulsive field."</ref>
In 1928, Walter Bothe observed that beryllium emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles. It was later discovered that this radiation could knock hydrogen atoms out of paraffin wax. Initially it was thought to be high-energy gamma radiation, since gamma radiation had a similar effect on electrons in metals, but James Chadwick found that the ionization effect was too strong for it to be due to electromagnetic radiation, so long as energy and momentum were conserved in the interaction. In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton. Chadwick called this new particle "the neutron" and believed that it to be a proton and electron fused together because the neutron had about the same mass as a proton and an electron's mass is negligible by comparison.<ref>Template:Cite journal</ref> Neutrons are not in fact a fusion of a proton and an electron.Template:Cn
See also
Template:Portal Template:Div col
- Spectroscopy
- Atom
- History of molecular theory
- Discovery of chemical elements
- Introduction to quantum mechanics
- Kinetic theory of gases
- Atomism
- The Physical Principles of the Quantum Theory
Footnotes
Template:Notelist Template:Reflist
Bibliography
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
- Template:Cite book
Further reading
- Charles Adolphe Wurtz (1881) The Atomic Theory, D. Appleton and Company, New York.
- Alan J. Rocke (1984) Chemical Atomism in the Nineteenth Century: From Dalton to Cannizzaro, Ohio State University Press, Columbus (open access full text at http://digital.case.edu/islandora/object/ksl%3Ax633gj985).
External links
- Atomism by S. Mark Cohen.
- Atomic Theory – detailed information on atomic theory with respect to electrons and electricity.
- The Feynman Lectures on Physics Vol. I Ch. 1: Atoms in Motion
Template:History of physics Template:History of chemistry Template:Atomic models