J. J. Thomson
Template:Short description Template:Other people Template:Use British English Template:Use dmy dates Template:Infobox officeholder
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist whose study of cathode rays led to his discovery of the electron, a subatomic particle with a negative electric charge.<ref name=NobelBio>Template:Cite web</ref> In 1897, he showed that cathode rays were composed of previously unknown negatively charged particles (now called electrons), which he calculated must have bodies much smaller than atoms and a very large charge-to-mass ratio.<ref name="Profile" />
In 1906, Thomson was awarded the Nobel Prize in Physics "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases."<ref name=Nobel1906>Template:Cite web</ref>
Thomson is credited with finding the first evidence for isotopes of a stable (non-radioactive) element in 1912, as part of his exploration into the composition of canal rays (positive ions). His experiments to determine the nature of positively charged particles, with Francis William Aston, were the first use of mass spectrometry and led to the development of the mass spectrograph.<ref name="Profile" /><ref name="Jones" />
Thomson was an influential teacher, and seven of his students went on to win Nobel Prizes: Ernest Rutherford (Chemistry 1908), Lawrence Bragg (Physics 1915), Charles Barkla (Physics 1917), Francis Aston (Chemistry 1922), Charles Thomson Rees Wilson (Physics 1927), Owen Richardson (Physics 1928) and Edward Appleton (Physics 1947).<ref>Template:Cite web</ref>
Biography
Joseph John Thomson was born on 18 December 1856 in Cheetham Hill, Manchester. His mother, Emma Swindells, came from a local textile family. His father, Joseph James Thomson, ran an antiquarian bookshop founded by Thomson's great-grandfather. Joseph John had a brother, Frederick Vernon Thomson, who was two years younger than he was.<ref name="ReferenceA">Davis & Falconer, J.J. Thomson and the Discovery of the Electron</ref> Thomson was a reserved yet devout Anglican.<ref>Peter J. Bowler, Reconciling Science and Religion: The Debate in Early-Twentieth-Century Britain (2014). University of Chicago Press. p. 35. Template:ISBN. "Both Lord Rayleigh and J. J. Thomson were Anglicans."</ref><ref>Seeger, Raymond. 1986. "J. J. Thomson, Anglican", in "Perspectives on Science and Christian Faith", 38 (June 1986): 131–132. The Journal of the American Scientific Affiliation. "As a Professor, J. J. Thomson did attend the Sunday evening college chapel service, and as Master, the morning service. He was a regular communicant in the Anglican Church. In addition, he showed an active interest in the Trinity Mission at Camberwell. With respect to his private devotional life, J. J. Thomson would invariably practice kneeling for daily prayer, and read his Bible before retiring each night. He truly was a practicing Christian!" (Raymond Seeger 1986, 132).</ref><ref>Richardson, Owen. 1970. "Joseph J. Thomson", in Dictionary of National Biography, 1931–1940. L. G. Wickham Legg, editor. Oxford University Press.</ref>
Education
Thomson's early education was in small private schools where he demonstrated outstanding talent and interest in science. In 1870, he was admitted to Owens College in Manchester (now the University of Manchester) at the unusually young age of 14, and came under the influence of Balfour Stewart, Professor of Physics, who initiated him into physical research.<ref>Template:Cite journal</ref> He began experimenting with contact electrification and soon published his first scientific paper.<ref>Template:Cite journal</ref> His parents planned to enroll him as an apprentice engineer to Sharp, Stewart & Co, a locomotive manufacturer, but these plans were cut short when his father died in 1873.<ref name="ReferenceA" />
In 1876, Thomson moved on to Trinity College, Cambridge. In 1880, he received his B.A. in mathematics (Second Wrangler in the Tripos<ref name="ThomsonProfile">Template:Cite web</ref> and 2nd Smith's Prizeman).<ref name="ACAD" /> He applied for and became a Fellow of Trinity College the following year.<ref name="Victoria">Template:Cite book Template:ISBN missing</ref> He obtained an M.A. (Adams Prizeman) in 1883.<ref name="ACAD">Template:Acad</ref>
Career
On 22 December 1884, Thomson was appointed Cavendish Professor at the University of Cambridge.<ref name="Profile">Template:Cite web</ref> This appointment caused considerable surprise; candidates such as Osborne Reynolds and Richard Glazebrook were older and more experienced in laboratory work, whereas Thomson was known for his work as a mathematician—being recognised as an exceptional talent.<ref name="Leadership">Template:Cite book</ref>
Thomson was knighted in 1908 and appointed to the Order of Merit in 1912.Template:Cn At Oxford, he gave the 1914 Romanes Lecture titled The Atomic Theory. In 1918, he became Master of Trinity College, Cambridge, a position he held until his death on 30 August 1940. His ashes rest in Westminster Abbey,<ref>'The Abbey Scientists' Hall, A.R. p. 63: London; Roger & Robert Nicholson; 1966</ref> near the graves of Isaac Newton and his former student, Ernest Rutherford.<ref name="sirJJrestingplace">Template:Cite web</ref>
Rutherford succeeded him as Cavendish Professor. Six of Thomson's research assistants and junior colleagues (Charles Glover Barkla,<ref>Template:Cite web</ref> Niels Bohr,<ref>Template:Cite web</ref> Max Born,<ref>Template:Cite web</ref> William Henry Bragg, Owen Willans Richardson<ref>Template:Cite encyclopedia</ref> and Charles Thomson Rees Wilson<ref name="frs">Template:Cite journal</ref>) won the Nobel Prize in Physics, and two (Francis William Aston<ref>Template:Cite web</ref> and Ernest Rutherford<ref name="nobelprize">Template:Cite web</ref>) won the Nobel Prize in Chemistry. Thomson's son, George Paget Thomson, won the 1937 Nobel Prize in Physics for proving the wave-like properties of electrons.<ref>Template:Cite web</ref>
Research
Early work
Thomson's prize-winning master's work, Treatise on the motion of vortex rings, shows his early interest in atomic structure.<ref name=NobelBio/> In it, Thomson mathematically described the motions of Lord Kelvin's vortex theory of the atom.<ref name="Leadership" />
Thomson published a number of papers addressing both mathematical and experimental issues of electromagnetism. He examined the electromagnetic theory of light of James Clerk Maxwell, introduced the concept of electromagnetic mass of a charged particle, and demonstrated that a moving charged body would apparently increase in mass.<ref name="Leadership" />
Much of his work in mathematical modelling of chemical processes can be thought of as early computational chemistry.<ref name="Profile" /> In further work, published in book form as Applications of dynamics to physics and chemistry (1888), Thomson addressed the transformation of energy in mathematical and theoretical terms, suggesting that all energy might be kinetic.<ref name="Leadership" /> His next book, Notes on recent researches in electricity and magnetism (1893), built upon Maxwell's Treatise upon electricity and magnetism, and was sometimes referred to as "the third volume of Maxwell."<ref name=NobelBio/> In it, Thomson emphasized physical methods and experimentation and included extensive figures and diagrams of apparatus, including a number for the passage of electricity through gases.<ref name="Leadership" /> His third book, Elements of the mathematical theory of electricity and magnetism (1895)<ref>Template:Cite journal</ref> was a readable introduction to a wide variety of subjects, and achieved considerable popularity as a textbook.<ref name="Leadership" />

A series of four lectures, given by Thomson on a visit to Princeton University in 1896, were subsequently published as Discharge of electricity through gases (1897). He also presented a series of six lectures at Yale University in 1904.<ref name=NobelBio/>
Discovery of the electron

Several scientists, such as William Prout and Norman Lockyer, had suggested that atoms were built up from a more fundamental unit, but they envisioned this unit to be the size of the smallest atom, hydrogen. Thomson in 1897 was the first to suggest that one of the fundamental units of the atom was more than 1,000 times smaller than an atom, suggesting the subatomic particle now known as the electron. Thomson discovered this through his explorations on the properties of cathode rays. Thomson made his suggestion on 30 April 1897 following his discovery that cathode rays (at the time known as Lenard rays) could travel much further through air than expected for an atom-sized particle.<ref name="referenceB">Template:Cite journal</ref> He estimated the mass of cathode rays by measuring the heat generated when the rays hit a thermal junction and comparing this with the magnetic deflection of the rays. His experiments suggested not only that cathode rays were over 1,000 times lighter than the hydrogen atom, but also that their mass was the same in whichever type of atom they came from. He concluded that the rays were composed of very light, negatively charged particles which were a universal building block of atoms. He called the particles "corpuscles", but later scientists preferred the name electron, which had been suggested by George Johnstone Stoney in 1891, prior to Thomson's discovery.<ref>Template:Cite book</ref>
In April 1897, Thomson had only early indications that the cathode rays could be deflected electrically (previous investigators such as Heinrich Hertz had thought they could not be). A month after Thomson's announcement of the corpuscle, he found that he could reliably deflect the rays by an electric field if he evacuated the discharge tube to a very low pressure. By comparing the deflection of a beam of cathode rays by electric and magnetic fields he obtained more robust measurements of the mass-to-charge ratio that confirmed his previous estimates.<ref name="PhilMag">Template:Cite journal</ref> This became the classic means of measuring the charge-to-mass ratio of the electron. Later in 1899 he measured the charge of the electron to be of Template:Val.<ref>Template:Cite journal</ref>
Thomson believed that the corpuscles emerged from the atoms of the trace gas inside his cathode-ray tubes. He thus concluded that atoms were divisible, and that the corpuscles were their building blocks. In 1904, Thomson suggested a model of the atom, hypothesizing that it was a sphere of positive matter within which electrostatic forces determined the positioning of the corpuscles.<ref name="Profile" /> To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge. In this "plum pudding model", the electrons were seen as embedded in the positive charge like raisins in a plum pudding (although in Thomson's model they were not stationary, but orbiting rapidly).<ref>Template:Citation</ref><ref>Template:Harvtxt, p. 324: "Thomson's model, then, consisted of a uniformly charged sphere of positive electricity (the pudding), with discrete corpuscles (the plums) rotating about the center in circular orbits, whose total charge was equal and opposite to the positive charge."</ref>
Thomson made the discovery around the same time that Walter Kaufmann and Emil Wiechert discovered the correct mass to charge ratio of these cathode rays (electrons).<ref>Template:Cite journal</ref>
The name electron was adopted for these particles by the scientific community, mainly due to the advocation by George Francis FitzGerald, Joseph Larmor, and Hendrik Lorentz.<ref name=OHara1975>
Template:Cite journal</ref>Template:Rp The term was originally coined by George Johnstone Stoney in 1891 as a tentative name for the basic unit of electrical charge (which had then yet to be discovered).<ref>Template:Cite journal</ref><ref>Template:Cite journal</ref> For some years Thomson resisted using the word "electron" because he didn't like how some physicists talked of a "positive electron" that was supposed to be the elementary unit of positive charge just as the "negative electron" is the elementary unit of negative charge. Thomson preferred to stick with the word "corpuscle" which he strictly defined as negatively charged.<ref>Template:Cite journal: "Perhaps I can best show my appreciation by trying to answer the questions which Professor Silvanus Thompson addressed to me. I think his first question was a question rather of notation, as to the difference between the electron and the corpuscle. I prefer the corpuscle for two reasons: first of all, it is my own child, and I have a kind of parental affection for it; and, secondly, I think it has one merit which the term electron has not. We talk about positive and negative electrons, and I think when you use the same term for the two the suggestion is that there is an equality, so to speak, in the properties. From my point of view the difference between the negative and the positive is essential, and much greater than I think would be suggested by the term positive electron and negative electron. Therefore I prefer to use a special term for the negative units and call it a corpuscle. A corpuscle is just a negative electron."</ref> He relented by 1914, using the word "electron" in his book The Atomic Theory.<ref>Template:Cite book</ref> In 1920, Rutherford and his fellows agreed to call the nucleus of the hydrogen ion "proton", establishing a distinct name for the smallest known positively-charged particle of matter (that can exist independently anyway).<ref>Template:Cite journalTemplate:Dead link
Footnote by Ernest Rutherford: 'At the time of writing this paper in Australia, Professor Orme Masson was not aware that the name "proton" had already been suggested as a suitable name for the unit of mass nearly 1, in terms of oxygen 16, that appears to enter into the nuclear structure of atoms. The question of a suitable name for this unit was discussed at an informal meeting of a number of members of Section A of the British Association [for the Advancement of Science] at Cardiff this year. The name "baron" suggested by Professor Masson was mentioned, but was considered unsuitable on account of the existing variety of meanings. Finally the name " proton" met with general approval, particularly as it suggests the original term "protyle " given by Prout in his well-known hypothesis that all atoms are built up of hydrogen. The need of a special name for the nuclear unit of mass 1 was drawn attention to by Sir Oliver Lodge at the Sectional meeting, and the writer then suggested the name "proton."'</ref>
Isotopes and mass spectrometry

In 1912, as part of his exploration into the composition of the streams of positively charged particles then known as canal rays, Thomson and his research assistant, F. W. Aston, channelled a stream of neon ions through a magnetic and an electric field and measured its deflection by placing a photographic plate in its path.<ref name="ReferenceA" /> They observed two patches of light on the photographic plate (see image on right), which suggested two different parabolas of deflection, and concluded that neon is composed of atoms of two different atomic masses (neon-20 and neon-22), that is to say of two isotopes.<ref>J.J. Thomson (1912) "Further experiments on positive rays," Philosophical Magazine, series 6, 24 (140): 209–253.</ref><ref>J. J. Thomson (1913) "Rays of positive electricity", Proceedings of the Royal Society A, 89: 1–20.</ref> This was the first evidence for isotopes of a stable element; Frederick Soddy had previously proposed the existence of isotopes to explain the decay of certain radioactive elements.
Thomson's separation of neon isotopes by their mass was the first example of mass spectrometry, which was subsequently improved and developed into a general method by F. W. Aston and by A. J. Dempster.<ref name="Profile" /><ref name="Jones">Template:Cite web</ref>
Experiments with cathode rays
Earlier, physicists debated whether cathode rays were immaterial like light ("some process in the aether") or were "in fact wholly material, and ... mark the paths of particles of matter charged with negative electricity", quoting Thomson.<ref name="PhilMag" /> The aetherial hypothesis was vague,<ref name="PhilMag" /> but the particle hypothesis was definite enough for Thomson to test.
Magnetic deflection
Thomson first investigated the magnetic deflection of cathode rays. Cathode rays were produced in the side tube on the left of the apparatus and passed through the anode into the main bell jar, where they were deflected by a magnet. Thomson detected their path by the fluorescence on a squared screen in the jar. He found that whatever the material of the anode and the gas in the jar, the deflection of the rays was the same, suggesting that the rays were of the same form whatever their origin.<ref>Template:Cite journal</ref>
Electrical charge

While supporters of the aetherial theory accepted the possibility that negatively charged particles are produced in Crookes tubes,Template:Citation needed they believed that they are a mere by-product and that the cathode rays themselves are immaterial.Template:Citation needed Thomson set out to investigate whether or not he could actually separate the charge from the rays.
Thomson constructed a Crookes tube with an electrometer set to one side, out of the direct path of the cathode rays. Thomson could trace the path of the ray by observing the phosphorescent patch it created where it hit the surface of the tube. Thomson observed that the electrometer registered a charge only when he deflected the cathode ray to it with a magnet. He concluded that the negative charge and the rays were one and the same.<ref name="referenceB"/> Template:Clear
Electrical deflection
Template:More citations needed Template:Multiple image

In May–June 1897, Thomson investigated whether or not the rays could be deflected by an electric field.<ref name="ReferenceA"/> Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because their tubes contained too much gas.
Thomson constructed a Crookes tube with a better vacuum. At the start of the tube was the cathode from which the rays projected. The rays were sharpened to a beam by two metal slits – the first of these slits doubled as the anode, the second was connected to the earth. The beam then passed between two parallel aluminium plates, which produced an electric field between them when they were connected to a battery. The end of the tube was a large sphere where the beam would impact on the glass, created a glowing patch. Thomson pasted a scale to the surface of this sphere to measure the deflection of the beam. Any electron beam would collide with some residual gas atoms within the Crookes tube, thereby ionizing them and producing electrons and ions in the tube (space charge); in previous experiments this space charge electrically screened the externally applied electric field. However, in Thomson's Crookes tube the density of residual atoms was so low that the space charge from the electrons and ions was insufficient to electrically screen the externally applied electric field, which permitted Thomson to successfully observe electrical deflection.
When the upper plate was connected to the negative pole of the battery and the lower plate to the positive pole, the glowing patch moved downwards, and when the polarity was reversed, the patch moved upwards. Template:Clear
Measurement of mass-to-charge ratio
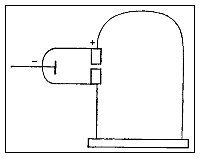
In his classic experiment, Thomson measured the mass-to-charge ratio of the cathode rays by measuring how much they were deflected by a magnetic field and comparing this with the electric deflection. He used the same apparatus as in his previous experiment, but placed the discharge tube between the poles of a large electromagnet. He found that the mass-to-charge ratio was over a thousand times lower than that of a hydrogen ion (H+), suggesting either that the particles were very light and/or very highly charged.<ref name="PhilMag"/> Significantly, the rays from every cathode yielded the same mass-to-charge ratio. This is in contrast to anode rays (now known to arise from positive ions emitted by the anode), where the mass-to-charge ratio varies from anode-to-anode. Thomson himself remained critical of what his work established, in his Nobel Prize acceptance speech referring to "corpuscles" rather than "electrons".
Thomson's calculations can be summarised as follows (in his original notation, using F instead of E for the electric field and H instead of B for the magnetic field):
The electric deflection is given by <math>\Theta = Fel / mv^2</math>, where Θ is the angular electric deflection, F is applied electric intensity, e is the charge of the cathode ray particles, l is the length of the electric plates, m is the mass of the cathode ray particles and v is the velocity of the cathode ray particles. The magnetic deflection is given by <math>\phi = Hel / mv</math>, where φ is the angular magnetic deflection and H is the applied magnetic field intensity.
The magnetic field was varied until the magnetic and electric deflections were the same, when <math>\Theta = \phi, Fel / mv^2 = Hel / mv</math>. This can be simplified to give <math>m/e = H^2 l/F\Theta</math>. The electric deflection was measured separately to give Θ and H, F and l were known, so m/e could be calculated. Template:Clear
Conclusions
As to the source of these particles, Thomson believed they emerged from the molecules of gas in the vicinity of the cathode.
Thomson imagined the atom as being made up of these corpuscles orbiting in a sea of positive charge; this was his plum pudding model. This model was later proved incorrect when his student Ernest Rutherford showed that the positive charge is concentrated in the nucleus of the atom.
Other work
In 1905, Thomson discovered the natural radioactivity of potassium.<ref name='Phil Mag 1905'>Template:Cite journal</ref>
In 1906, Thomson demonstrated that hydrogen had only a single electron per atom. Previous theories allowed various numbers of electrons.<ref>Template:The Timetables of Science</ref><ref name='Phil Mag 1906'>Template:Cite journal</ref>
From 1916 to 1918, Thomson chaired the "Committee appointed by the Prime Minister to enquire into the Position of Natural Science in the Educational System of Great Britain". The Report of the Committee, published in 1918, was known as the Thomson Report.<ref>Template:Cite web</ref>
Family
In 1890, Thomson married Rose Elisabeth Paget at the church of St. Mary the Less. Rose, who was the daughter of Sir George Edward Paget, a physician and then Regius Professor of Physic at Cambridge, was interested in physics. Beginning in 1882, women could attend demonstrations and lectures at the University of Cambridge. Rose attended demonstrations and lectures, among them Thomson's, leading to their relationship.<ref>Template:Cite book</ref>
They had two children: George Paget Thomson, who was also awarded a Nobel Prize for his work on the wave properties of the electron; and Joan Paget Thomson (later Charnock),<ref>Template:Cite web</ref> who became an author—writing children's books, non-fiction, and biographies.<ref>Template:Cite book</ref>
Recognition
Memberships
| Country | Year | Institute | Type | Template:Reference column heading |
|---|---|---|---|---|
| Template:Flagdeco United Kingdom | 1884 | Royal Society | Fellow | <ref>Template:Cite web</ref> |
| Template:Flag | 1902 | American Academy of Arts and Sciences | International Honorary Member | <ref>Template:Cite web</ref> |
| Template:Flag | 1903 | American Philosophical Society | International Member | <ref>Template:Cite web</ref> |
| Template:Flag | 1903 | National Academy of Sciences | International Member | <ref>Template:Cite web</ref> |
Awards
| Country | Year | Institute | Award | Citation | Template:Reference column heading |
|---|---|---|---|---|---|
| Template:Flagdeco United Kingdom | 1894 | Royal Society | Royal Medal | "For his contributions to mathematical and experimental physics, especially to electrical theory" | <ref>Template:Cite web</ref> |
| Template:Flag | 1902 | Smithsonian Institution | Hodgkins Medal | <ref>Template:Cite web</ref> | |
| Template:Flagdeco United Kingdom | 1902 | Royal Society | Hughes Medal | "For his numerous contributions to electric science, especially in reference to the phenomena of electric discharge in gases" | <ref>Template:Cite web</ref> |
| Template:Flag | 1906 | Royal Swedish Academy of Sciences | Nobel Prize in Physics | "In recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases" | <ref name=Nobel1906/> |
| Template:Flag | 1910 | Franklin Institute | Elliott Cresson Medal | "For distinguished work in physical sciences" | <ref>Template:Cite web</ref> |
| Template:Flagdeco United Kingdom | 1914 | Royal Society | Copley Medal | "On the ground of his discoveries in physical science" | <ref>Template:Cite web</ref> |
| Template:Flagdeco United Kingdom | 1915 | Royal Society of Arts | RSA Albert Medal | <ref>Template:Cite web</ref> | |
| Template:Flag | 1922 | Franklin Institute | Franklin Medal | "For service as teacher and leader in electricity and the constitution of matter" | <ref>Template:Cite web</ref> |
| Template:Flag | 1925 | Institution of Electrical Engineers | IEE Faraday Medal | <ref>Template:Cite web</ref> |
Commemorations
In November 1927, Thomson opened the Thomson building, named in his honour, in the Leys School, Cambridge.<ref>Template:Cite web</ref>
In 1991, the thomson (symbol: Th) was proposed as a unit to measure mass-to-charge ratio in mass spectrometry in his honour.<ref>Template:Cite journal</ref>
J J Thomson Avenue, on the University of Cambridge's West Cambridge site, is named after Thomson.<ref>Template:Cite web</ref>
The Thomson Medal Award, sponsored by the International Mass Spectrometry Foundation, is named after Thomson.<ref>Template:Cite web</ref>
The Institute of Physics Joseph Thomson Medal and Prize is named after Thomson.<ref>Template:Cite web</ref>
Thomson Crescent in Deep River, Ontario, connects with Rutherford Ave. Template:Clear
See also
References
Bibliography


- 1883. A Treatise on the Motion of Vortex Rings: An essay to which the Adams Prize was adjudged in 1882, in the University of Cambridge. London: Macmillan and Co., pp. 146. Recent reprint: Template:ISBN.
- 1888. Applications of Dynamics to Physics and Chemistry. London: Macmillan and Co., pp. 326. Recent reprint: Template:ISBN.
- 1893. Notes on recent researches in electricity and magnetism: intended as a sequel to Professor Clerk-Maxwell's 'Treatise on Electricity and MagnetismTemplate:'. Oxford University Press, pp. xvi & 578. 1991, Cornell University Monograph: Template:ISBN.
- Template:Cite book
- Template:Cite book
- Thomson, Joseph John (1904). Electricity and matter (in English). Oxford : Clarendon Press.
- Template:Cite book
- Template:Cite book
- 1921 (1895). Elements of the Mathematical Theory of Electricity And Magnetism. London: Macmillan and Co. Scan of 1895 edition.
- A Text book of Physics in Five Volumes, co-authored with J.H. Poynting: (1) Properties of Matter, (2) Sound, (3) Heat, (4) Light, and (5) Electricity and Magnetism. Dated 1901 and later, and with revised later editions.
- Template:Cite book
- J.J. Thomson (1897) "Cathode Rays", The Electrician 39, 104, also published in Proceedings of the Royal Institution 30 April 1897, 1–14 – first announcement of the "corpuscle" (before the classic mass and charge experiment)
- J.J. Thomson (1897), Cathode rays, Philosophical Magazine, 44, 293 – the classic measurement of the electron mass and charge
- J.J. Thomson (1904), "On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure," Philosophical Magazine Series 6, Volume 7, Number 39, pp. 237–265. This paper presents the classical "plum pudding model" from which the Thomson Problem is posed.
- Template:Cite journal
- Template:Cite book

Corpuscular theory of matter, 1908 - J.J. Thomson (1912), "Further experiments on positive rays" Philosophical Magazine, 24, 209–253 – first announcement of the two neon parabolae
- J.J. Thomson (1913), Rays of positive electricity, Proceedings of the Royal Society, A 89, 1–20 – discovery of neon isotopes
- J.J. Thomson (1923), The Electron in Chemistry: Being Five Lectures Delivered at the Franklin Institute, Philadelphia.
- Thomson, Sir J. J. (1936), Recollections and Reflections, London: G. Bell & Sons, Ltd. Republished as digital edition, Cambridge: University Press, 2011 (Cambridge Library Collection series).
- Thomson, George Paget. (1964) J.J. Thomson: Discoverer of the Electron. Great Britain: Thomas Nelson & Sons, Ltd.
- Davis, Eward Arthur & Falconer, Isobel (1997), J.J. Thomson and the Discovery of the Electron. Template:ISBN
- Falconer, Isobel (1988) "J.J. Thomson's Work on Positive Rays, 1906–1914" Historical Studies in the Physical and Biological Sciences 18(2) 265–310
- Falconer, Isobel (2001) "Corpuscles to Electrons" in J Buchwald and A Warwick (eds) Histories of the Electron, Cambridge, Mass: MIT Press, pp. 77–100.
- Template:Cite journal
- Template:Cite journal
External links
Template:Library resources box Template:Commons Template:Wikisource Template:Wikiquote
- The Discovery of the Electron Template:Webarchive
- Template:Nobelprize with the Nobel Lecture, 11 December 1906 Carriers of Negative Electricity
- Annotated bibliography for Joseph J. Thomson from the Alsos Digital Library for Nuclear Issues
- Essay on Thomson life and religious views
- The Cathode Ray Tube site
- Thomson's discovery of the isotopes of Neon
- Photos of some of Thomson's remaining apparatus at the Cavendish Laboratory Museum
- A short film of Thomson lecturing on electrical engineering and the discovery of the electron (1934)
- Template:Gutenberg author
- Template:Internet Archive author
- A history of the electron: JJ and GP Thomson published by the University of the Basque Country (2013)
Template:S-start Template:S-aca Template:S-bef Template:S-ttl Template:S-aft Template:S-bef Template:S-ttl Template:S-aft Template:S-break Template:S-npo Template:S-bef Template:S-ttl Template:S-aft Template:S-end
Template:Copley Medallists 1901-1950 Template:Nobel Prize in Physics Laureates 1901-1925 Template:1906 Nobel Prize winners Template:Royal Society presidents 1900s Template:Scientists whose names are used as non SI units Template:Scientists whose names are used in physical constants Template:Masters of Trinity College, Cambridge Template:Dalton Medallists Template:Portal bar Template:Authority control
- 1856 births
- 1940 deaths
- 20th-century British physicists
- Alumni of Trinity College, Cambridge
- Burials at Westminster Abbey
- English Anglicans
- 20th-century British mathematicians
- British Nobel laureates
- British experimental physicists
- Fellows of the Royal Society
- Foreign associates of the National Academy of Sciences
- Masters of Trinity College, Cambridge
- Members of the Order of Merit
- Nobel laureates in Physics
- People from Cheetham Hill
- Presidents of the Royal Society
- Recipients of the Copley Medal
- Royal Medal winners
- Knights Bachelor
- Second Wranglers
- Alumni of the Victoria University of Manchester
- Presidents of the British Science Association
- Presidents of the Institute of Physics
- Presidents of the Physical Society
- Mass spectrometrists
- Recipients of the Dalton Medal
- Cavendish Professors of Physics
- Recipients of Franklin Medal
- International members of the American Philosophical Society
- Presidents of the Cambridge Philosophical Society